QUALITY OVERALL SUMMARY - CHEMICAL ENTITIES MOCK-UP ("QOS-CE MOCK-UP") (version 2005-05-24) NOTE TO READER: The follow
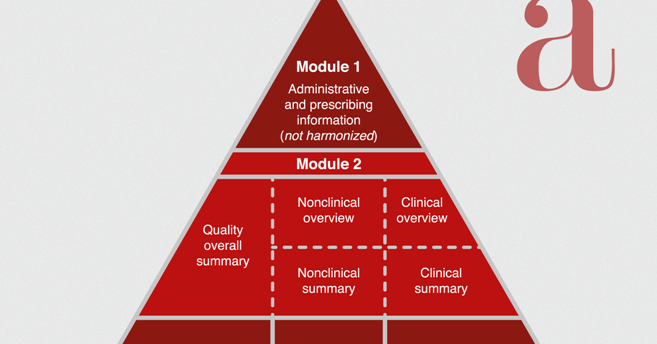
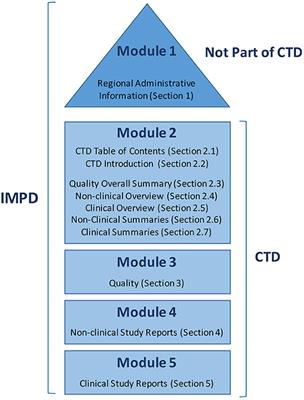
Brand name> 1 QOS-CE (NDS/ANDS) (2004-04-01) MODULE 2.3: QUALITY OVERALL SUMMARY (QOS) INTRODUCTION (a) Summary of produc
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

The Challenge of CTD Submissions and Responding to Questions from the Authorities - Trilogy Writing & Consulting GmbH
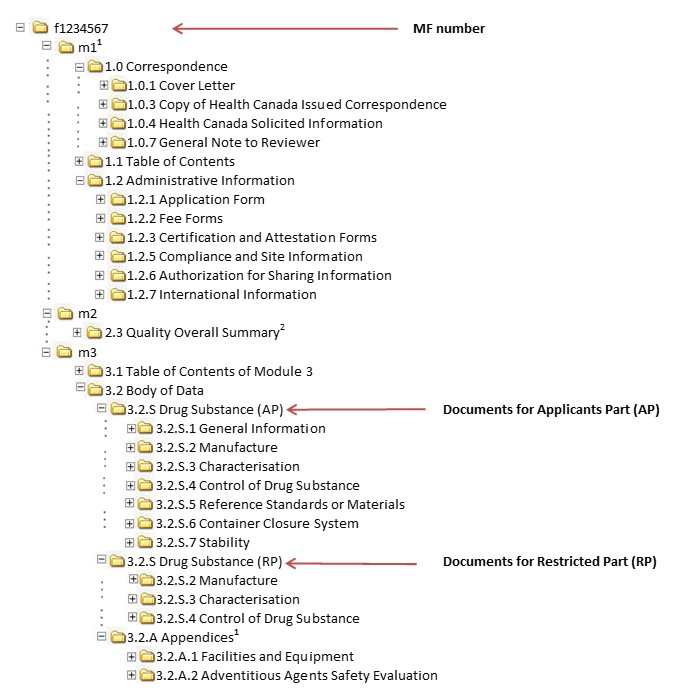
Between Standardisation and Flexibility – Defining Granularity of the eCTD Module 3.2.S for Different Types of Drug Substan