Prognostic Value of 18F-FDG PET/CT in a Large Cohort of Patients with Advanced Metastatic Neuroendocrine Neoplasms Treated with Peptide Receptor Radionuclide Therapy | Journal of Nuclear Medicine
![Use of [177Lu]Lu-DOTA-TATE in the treatment of gastroenteropancreatic neuroendocrine tumours: Results of a UK cost-effectiveness modelling study - ScienceDirect Use of [177Lu]Lu-DOTA-TATE in the treatment of gastroenteropancreatic neuroendocrine tumours: Results of a UK cost-effectiveness modelling study - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1359634921000033-gr1.jpg)
Use of [177Lu]Lu-DOTA-TATE in the treatment of gastroenteropancreatic neuroendocrine tumours: Results of a UK cost-effectiveness modelling study - ScienceDirect

Lutathera (Lutetium Lu 177 Dotatate) First Radioactive Drug Approved for Gastroenteropancreatic Neuroendocrine Tumors - Journal of Oncology Navigation & Survivorship
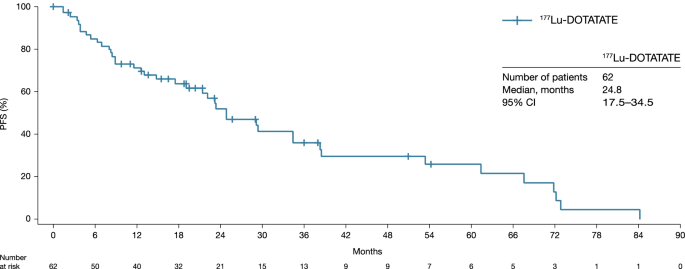
Efficacy and safety of 177Lu‑DOTATATE in patients with advanced pancreatic neuroendocrine tumours: data from the NETTER-R international, retrospective study | SpringerLink

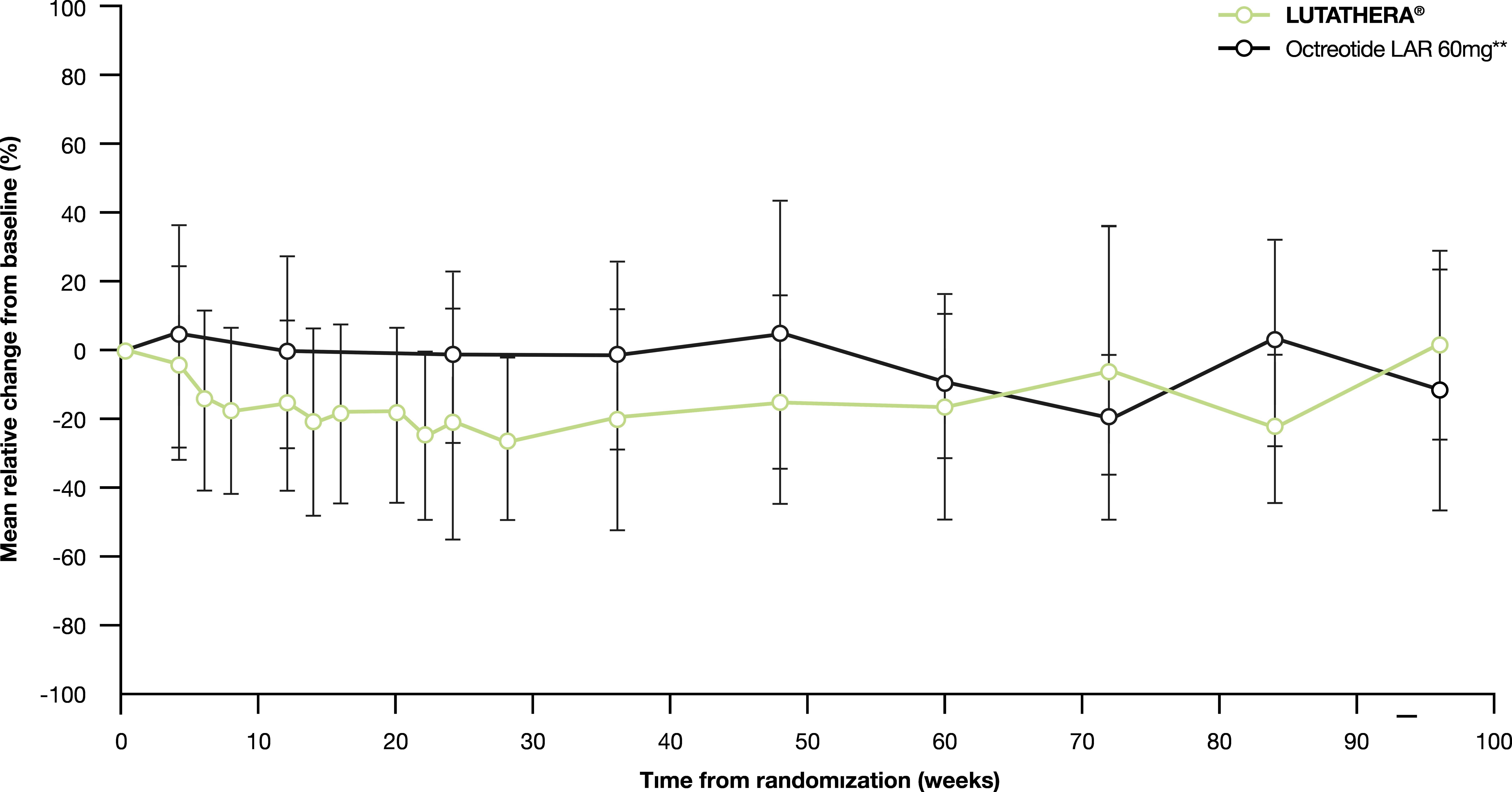
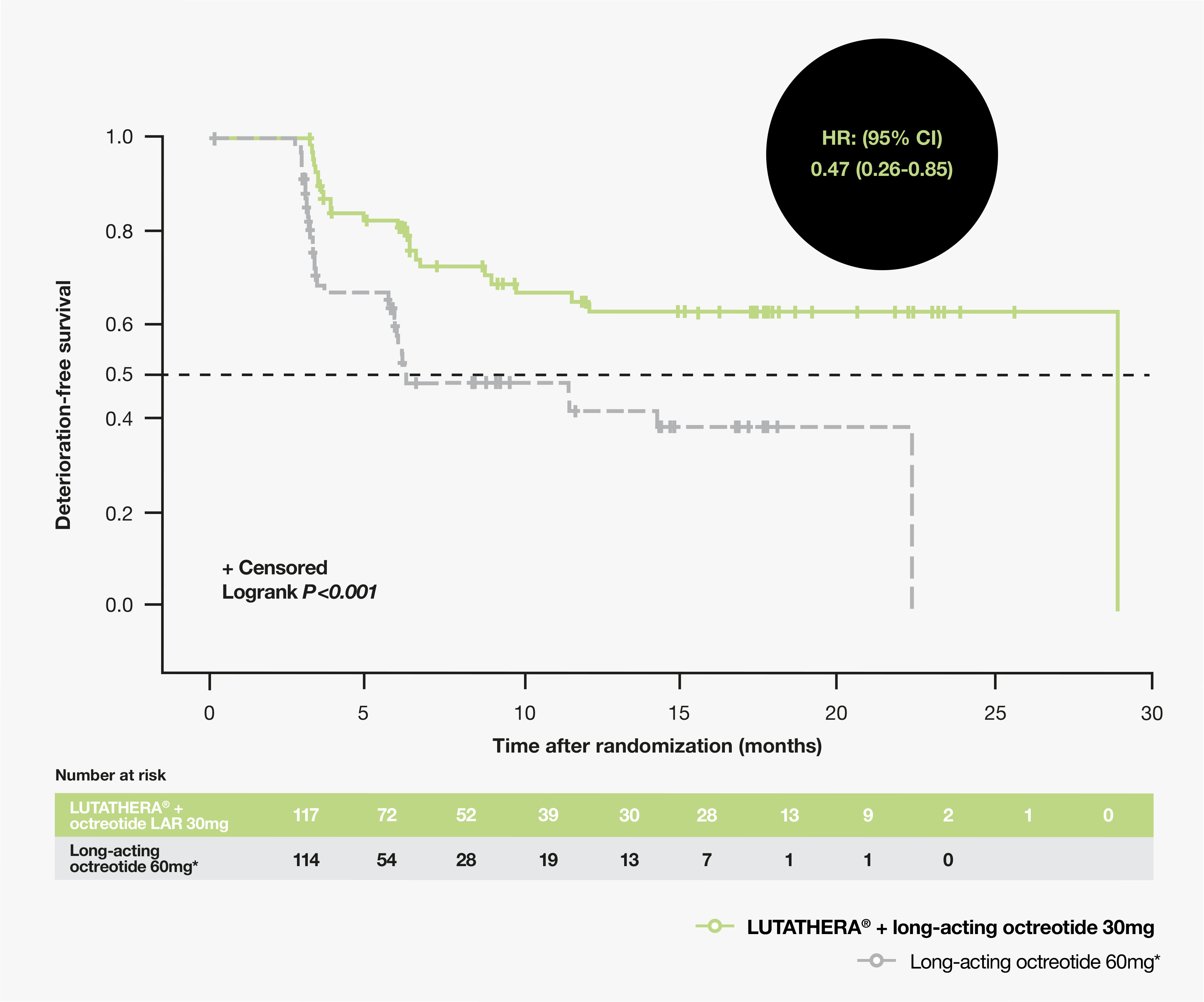
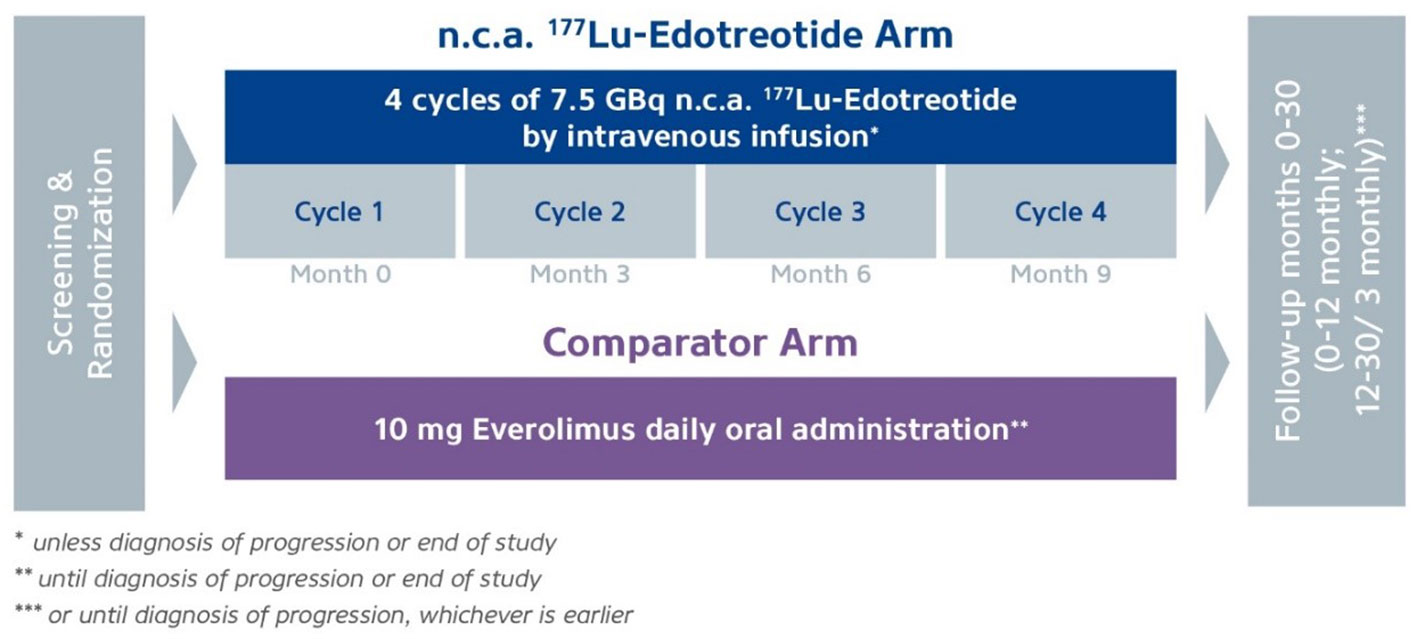
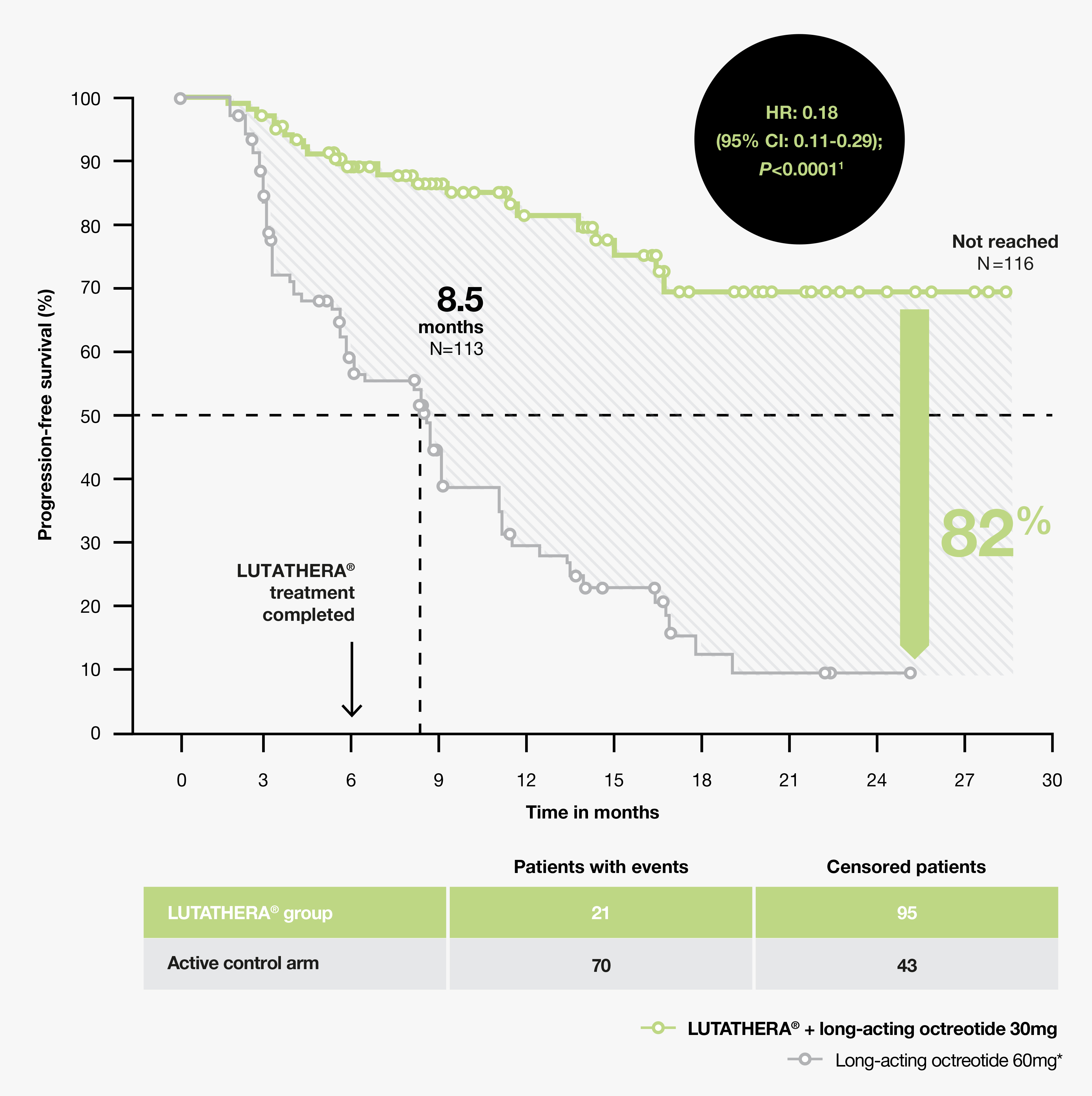
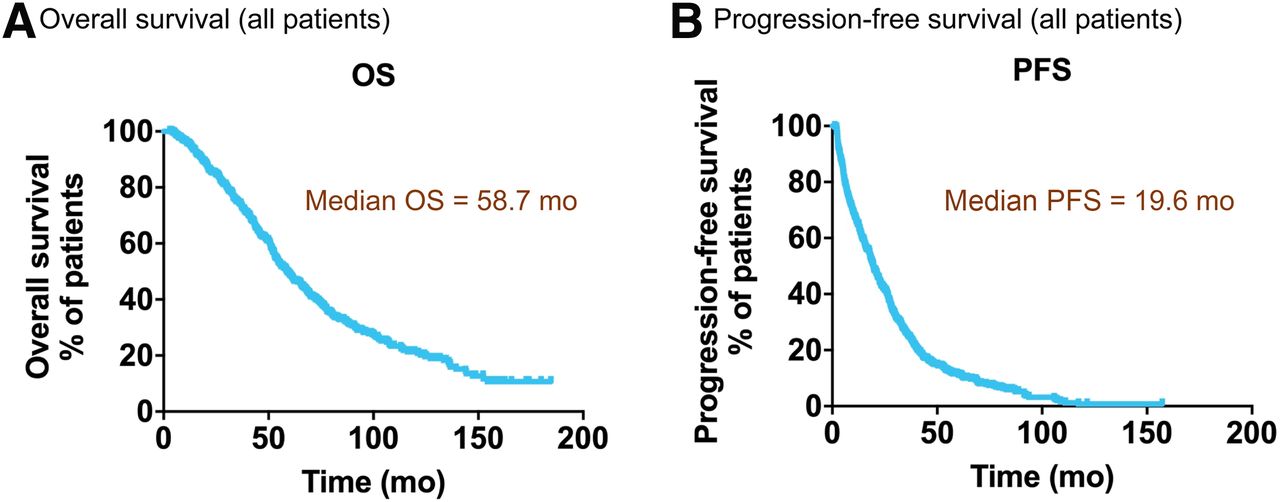
177Lu-Dotatate plus long-acting octreotide versus high‑dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial ...

Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up - ScienceDirect
Lutathera (lutetium Lu 177 dotatate) for the Treatment of Gastroenteropancreatic Neuroendocrine Tumours - Clinical Trials Arena
Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients