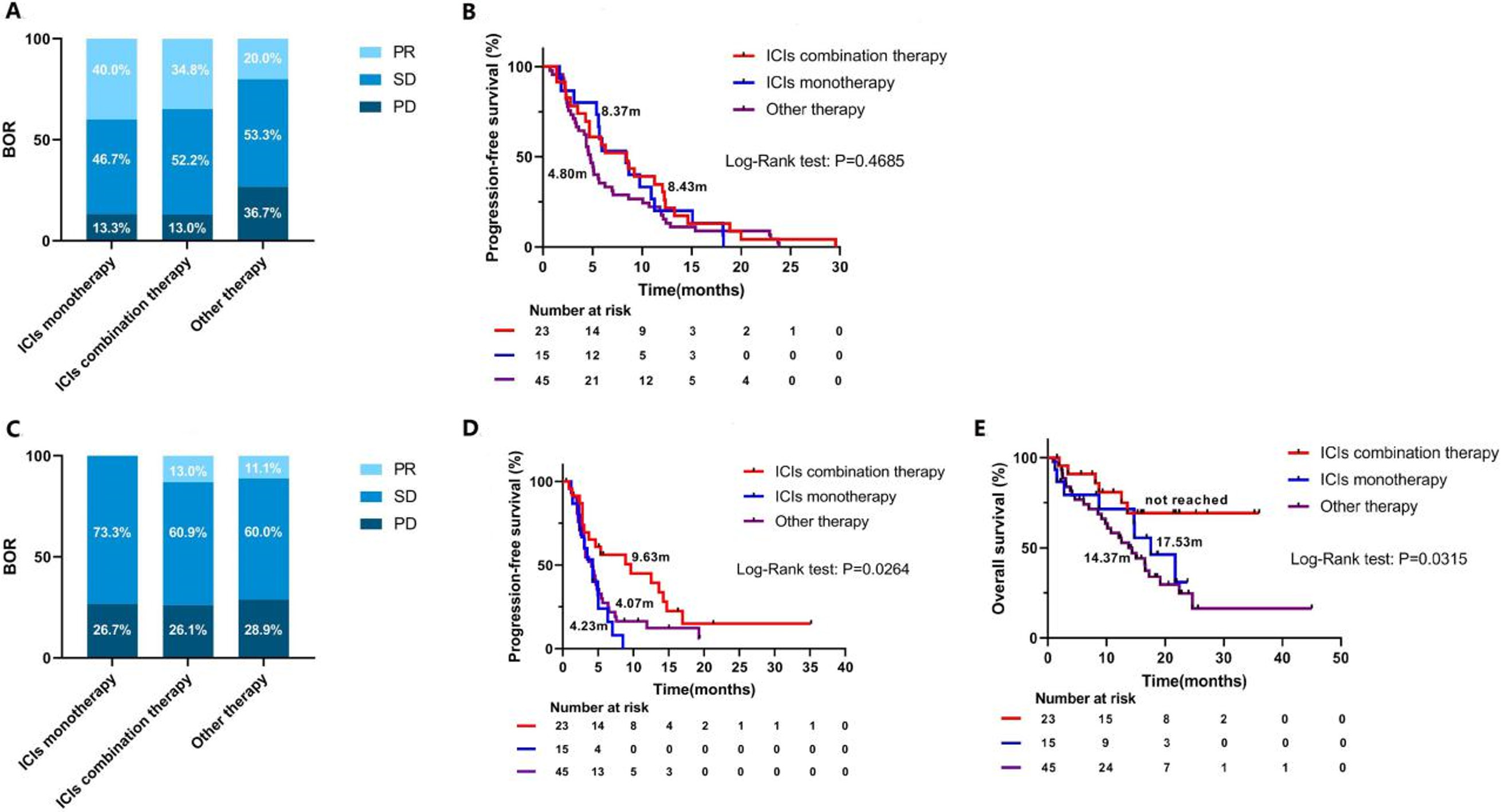
Favorable clinical outcomes of checkpoint inhibitor-based combinations after progression with immunotherapy in advanced non-small cell lung cancer
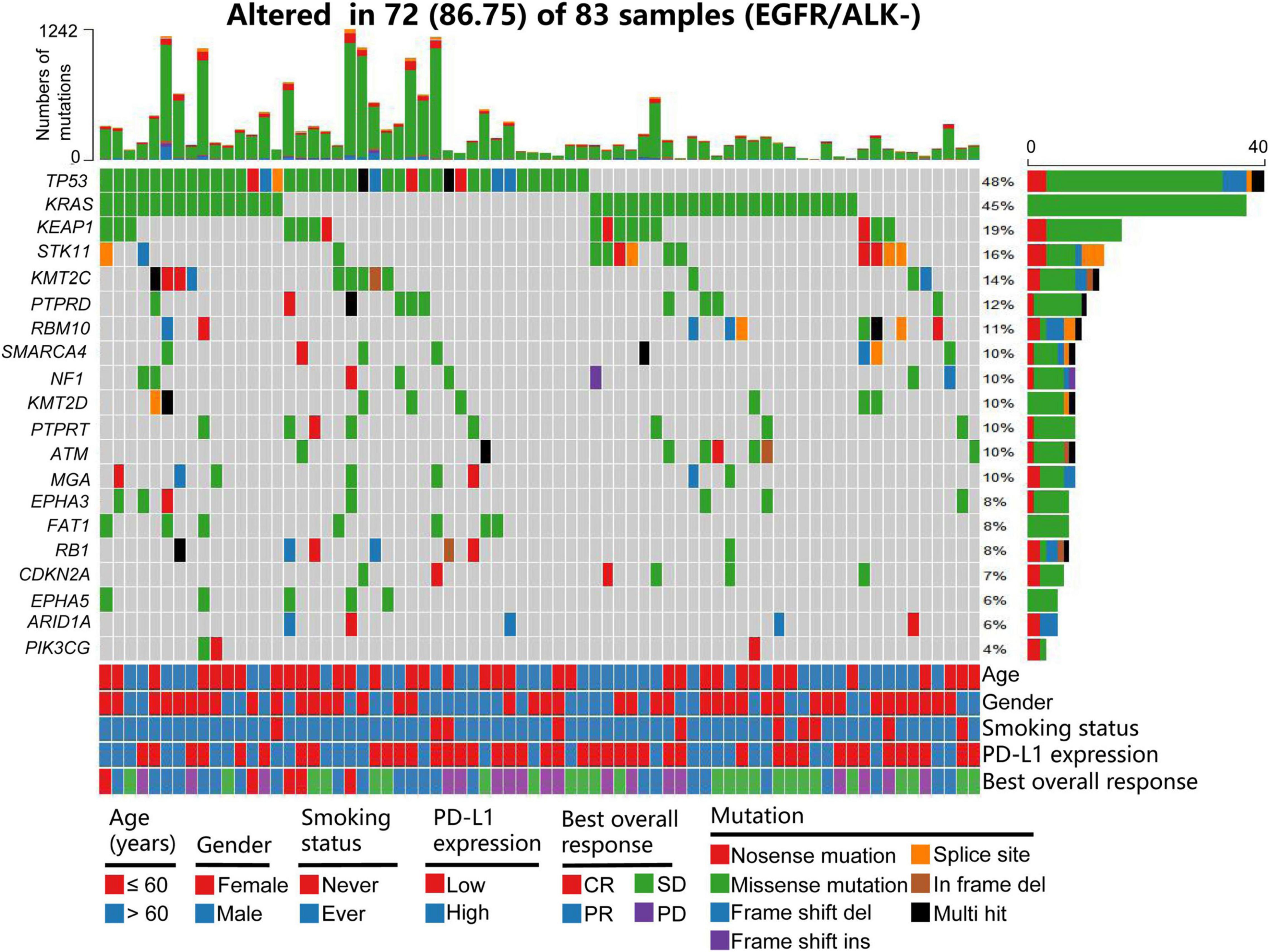
Frontiers | A Somatic Mutation Signature Predicts the Best Overall Response to Anti-programmed Cell Death Protein-1 Treatment in Epidermal Growth Factor Receptor/Anaplastic Lymphoma Kinase-Negative Non-squamous Non-small Cell Lung Cancer

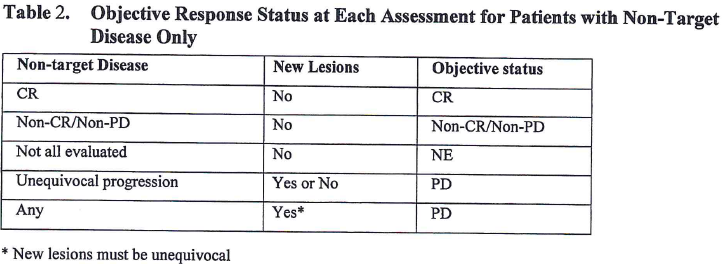
Table 3 from New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). | Semantic Scholar

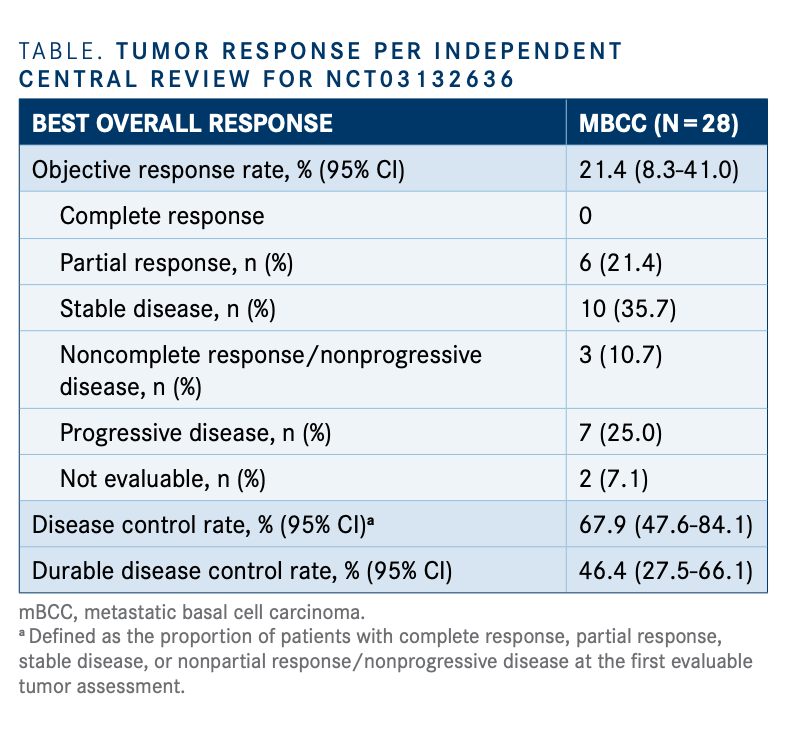
Best overall response (per RECIST 1.1, based on investigator assessment) | Download Scientific Diagram
Prepare ADaM data sets for Objective Response Rate (ORR) and Progression-Free Survival (PFS) analysis in an efficient way
Bevacizumab in Combination with Modified FOLFOX6 in Heavily Pretreated Patients with HER2/Neu-Negative Metastatic Breast Cancer: A Phase II Clinical Trial | PLOS ONE
Pan-phosphatidylinositol 3-kinase inhibition with buparlisib in patients with relapsed or refractory non-Hodgkin lymphoma | Haematologica
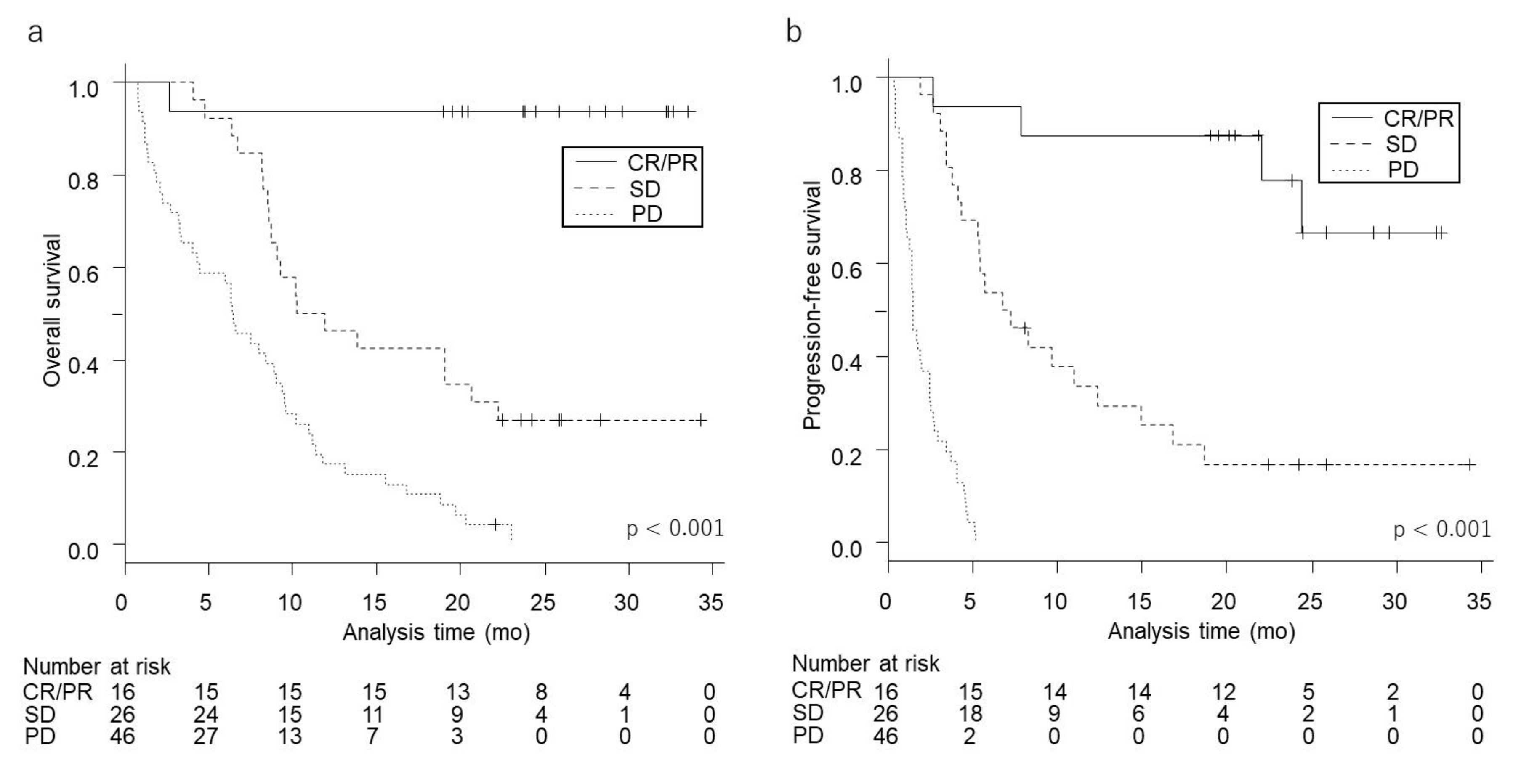
Cancers | Free Full-Text | Real-World, Long-Term Outcomes of Nivolumab Therapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck and Impact of the Magnitude of Best Overall Response:
Simplifying the Derivation of Best Overall Response per RECIST 1.1 and iRECIST in Solid Tumor Clinical Studies