
Tip Tuesday - 21 CFR Part 11 Electronic Records: Part 2 User Name and Password Limiting System Acces - YouTube

21 CFR Part 11, Interview Questions and Answers | Electronic Records & Signatures | PART 1 of 2 - YouTube

21 CFR Part 11 (Electronic Records/Signatures) Compliance for Computer Systems Regulated by FDA – Assent Pro

21 CFR Part 11 Electronic Signature | Learning management system, Electronic records, Good passwords

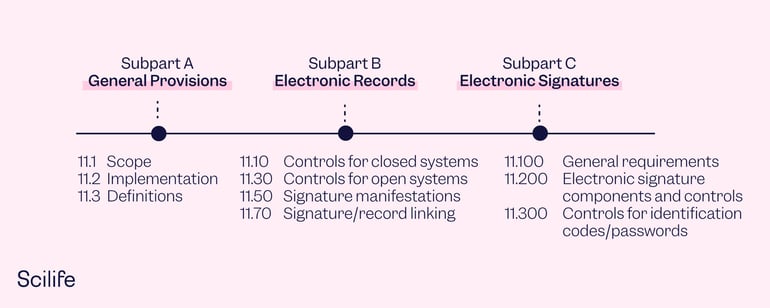



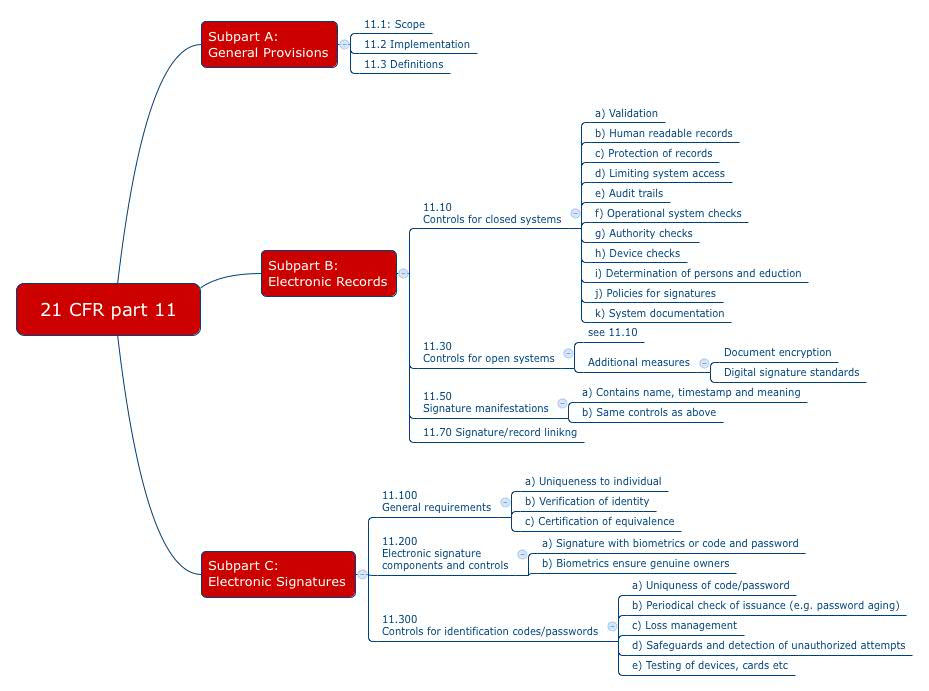


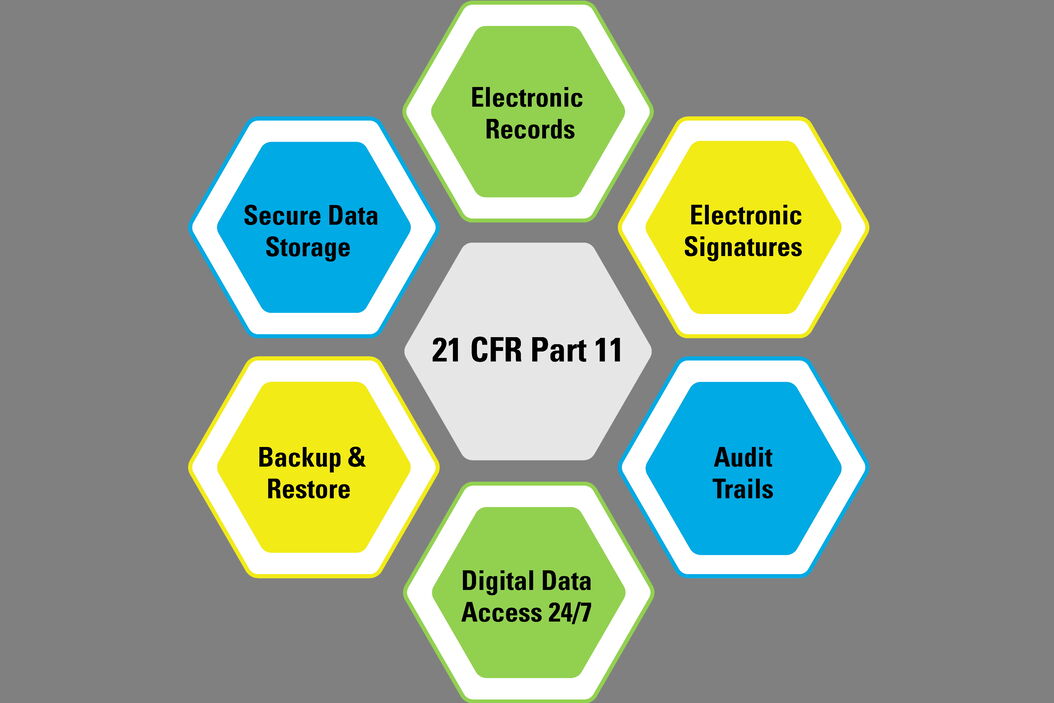






.png?width=2400&name=21-cfr-part%2011-guide%20(1).png)


